Laboratory Support:
SST (Salt Spray Test)
The salt spray test is a standardized test method used to check corrosion resistance of coated samples. Coatings provide corrosion resistance to metallic parts made of steel, zamak or brass. Since coatings can provide a high corrosion resistance through the intended life of the part in use, it is necessary to check corrosion resistance by other means. Salt spray test is an accelerated corrosion test that produces a corrosive attack to the coated samples in order to predict its suitability in use as a protective finish. The appearance of corrosion products (oxides) is evaluated after a period of time. Test duration depends on the corrosion resistance of the coating; the more corrosion resistant the coating is, the longer the period in testing without showing signs of corrosion.
Salt spray testing is popular because it is cheap, quick, well standardized and reasonably repeatable. There is, however, only a weak correlation between the duration in salt spray test and the expected life of a coating, since corrosion is a very complicated process and can be influenced by many external factors. Nevertheless, salt spray test is widely used in the industrial sector for the evaluation of corrosion resistance of finished surfaces or parts.
The instrument used in ultraviolet-visible spectroscopy is called a UV/vis spectrophotometer. It measures the intensity of light passing through a sample (I), and compares it to the intensity of light before it passes through the sample (Io). The ratio I / Io is called the transmittance, and is usually expressed as a percentage (%T). The absorbance, A, is based on the transmittance:
-
- A = − log(%T / 100%)
The basic parts of a spectrophotometer are a light source, a holder for the sample, a diffraction grating or monochromator to separate the different wavelengths of light, and a detector. The radiation source is often a Tungsten filament (300-2500 nm), a deuterium arc lamp which is continuous over the ultraviolet region (190-400 nm), and more recently light emitting diodes (LED) and Xenon Arc Lamps for the visible wavelengths. The detector is typically a photodiode or a CCD. Photodiodes are used with monochromators, which filter the light so that only light of a single wavelength reaches the detector. Diffraction gratings are used with CCDs, which collects light of different wavelengths on different pixels.
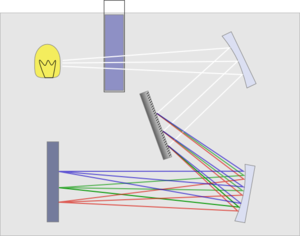
A spectrophotometer can be either single beam or double beam. In a single beam instrument (such as the Spectronic 20), all of the light passes through the sample cell. Io must be measured by removing the sample. This was the earliest design, but is still in common use in both teaching and industrial labs.
In a double-beam instrument, the light is split into two beams before it reaches the sample. One beam is used as the reference; the other beam passes through the sample. Some double-beam instruments have two detectors (photodiodes), and the sample and reference beam are measured at the same time. In other instruments, the two beams pass through a beam chopper, which blocks one beam at a time. The detector alternates between measuring the sample beam and the reference beam.
Samples for UV/Vis spectrophotometry are most often liquids, although the absorbance of gases and even of solids can also be measured. Samples are typically placed in a transparent cell, known as a cuvette. Cuvettes are typically rectangular in shape, commonly with an internal width of 1 cm. (This width becomes the path length, L, in the Beer-Lambert law.) Test tubes can also be used as cuvettes in some instruments. The type of sample container used must allow radiation to pass over the spectral region of interest. The most widely applicable cuvettes are made of high quality fused silica or quartz glass because these are transparent throughout the UV, visible and near infrared regions. Glass and plastic cuvettes are also common, although glass and most plastics absorb in the UV, which limits their usefulness to visible wavelengths.
The Hull Cell is a type of test cell used to qualitatively check the condition of a plating bath. It allows for optimization for current density range, optimization of additive concentration, recognition of impurity effects and indication of macro-throwing power capability. The Hull cell replicates the plating bath on a lab scale. It is filled with a sample of the plating solution, an appropriate anode which is connected to a rectifier. The "work" is replaced with a hull cell test panel that will be plated to show the "health" of the bath.

The Hull Cell is a trapezoidal container that holds 267 ml of solution. This shapes allows to place the test panel on an angle to the anode. As a result the deposit is plated at different current densities which can be measure with a hull cell ruler. The solution volume allows for a quantitative optimization of additive concentration: 1 gram addition to 267 mL is equivalent to 0.5 oz/gal in the plating tank.
Thickness Testing
Etc
- source: http://wikipedia.org/